The Oxford vaccine is safe in older adults and generates an immune response

The second phase of clinical trials of the COVID-19 vaccine developed by Oxford University in England has shown that it is safe in healthy older people and provokes an immune response, the medical journal The Lancet reports on Thursday.
In collaboration with pharmacist AstraZeneca and others, researchers tested the preparation, called ChAdOx1 nCoV-19, in an experiment with 560 healthy adults, including 240 over 70 years of age, to see how it impacts on the immune system and possible side effects.
The "promising preliminary results" indicate that this SARS-CoV-2 vaccine offers "similar safety and immunogenicity results in healthy older adults as in those aged 18-55 years".
According to The Lancet, Phase 2 concludes that the antidote causes "few side effects" and "induces an immune response in both parts of the immune system in all age groups at both low and standard doses".
According to the study, the British vaccine generates a response from T cells (capable of finding and attacking cells infected by the virus) 14 days after the first dose, and an antibody response 28 days after the booster dose (which would attack the virus when it circulates in the blood or lymphatic system).
The authors note that Phase 3 clinical trials, which are underway, should confirm these results and determine "how effective the vaccine is in protecting against SARS-CoV-2 infection" in a broader, heterogeneous group of people, including older people with previous pathologies.
In the widespread study, which does not measure the vaccine's effectiveness in protecting against the virus, 560 adults (160 aged 18-55; 160 aged 56-59 and 240 aged over 70) in good health received either the Oxford vaccine or a control antidote.

Volunteers over 55 years of age were divided into two groups and received either a single dose of the vaccine or two doses over a 28-day period. All were observed from the beginning for adverse effects as well as the immune response.
The authors note that side effects of ChAdOx1 nCoV-19 were "mild" (such as pain on injection, fatigue, headache, fever or muscle pain) although more common than with the control vaccine.
Thirteen cases of severity were detected within six months of the first dose, but these are not considered vaccine-related.
The researchers explain that side effects were even less common in older adults than in younger ones, and the immune response was "similar" in all age groups after the booster dose.
The vaccine induced antibodies to the coronavirus peak protein 28 days after a low or standard first dose in all age groups. After the booster vaccination, the antibody level increased 56 days after the start of the experiment, and the same was true for the neutralising antibodies 42 days later.
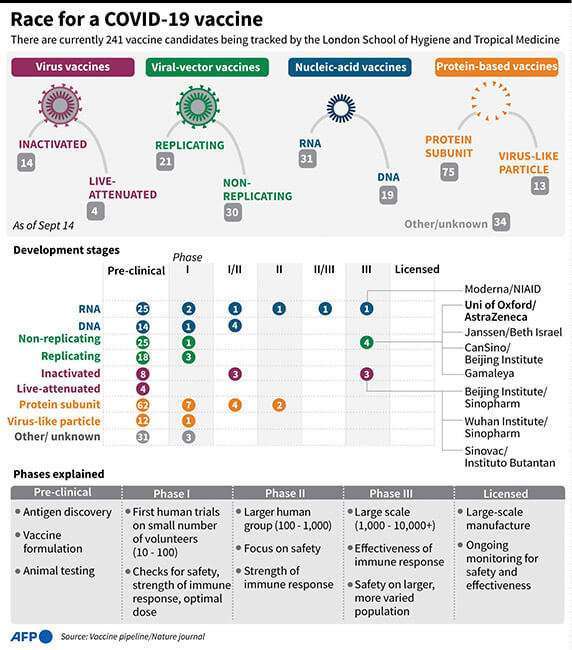
The response of T-cells against the coronavirus spike protein culminated 14 days after the first inoculation, regardless of age or dose.
Researcher Sarah Gilbert says that this study "answers some of the questions" raised by the World Health Organization (WHO) about the need for IDOC vaccines to protect older adults.
However, she points out, other "questions about efficacy and duration of protection" remain unanswered and the vaccine must also be tested in older people with pathologies to ensure that it protects those most at risk of serious illness.
The authors acknowledge that their experiment "has limitations", for example, that the older people had an average age of 73 or 74 and were healthy, which does not reflect the situation in nursing homes.
Furthermore, they add, the majority of volunteers of any age were white and non-smokers, so the third phase of the clinical trials will extend the testing to people from different backgrounds.








